Scientists: VPARP
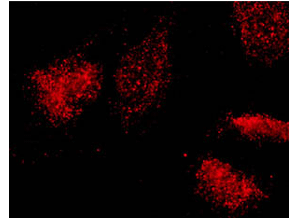
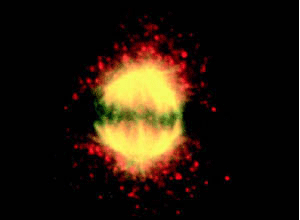
VPARP staining in HeLa cells reveals a punctate cytoplasmic pattern with some nuclear speckles (above left). A portion of VPARP co-localizes with beta tubulin to the mitotic spindle in dividing cells (yellow color above right).
The 193 kDa vault protein (p193) was recently identified by virtue of its interaction with the major vault protein in a yeast two-hybrid screen (Kickhoefer et al., 1999b). The identity of the clone was confirmed by peptide sequence analysis of a peptide derived from purified vault p193. Analysis of the p193 protein sequence revealed a region of ~350 amino acids that shares 28% identity with the catalytic domain of the enzyme poly (ADP-ribose) polymerase (PARP).
PARP is a well-characterized nuclear protein that catalyzes the formation of ADP ribose polymers onto specific proteins in response to DNA damage. The catalytic domain of p193 was expressed and purified from bacteria and found capable of catalyzing a poly ADP-ribosylation reaction. Thus the p193 protein is a new member of the PARP family of enzymes and it has been named VPARP to reflect this activity.
The catalytic domain of p193 carried out poly ADP-ribosylation of itself (autocatalysis) and purified vaults were also found to contain this activity. In addition to itself, the major vault protein appears to be a target of VPARP poly ADP-ribosylation.
Cell fractionation studies suggest that, like the vRNA and TEP1, VPARP is not localized solely to the vault particle. The protein also fractionates with the nuclear and cytosolic fractions. Immunofluorescence studies support this finding and reveal as well a VPARP association with the mitotic spindle in dividing cells (Kickhoefer et al., 1999b).