Scientists: TEP1
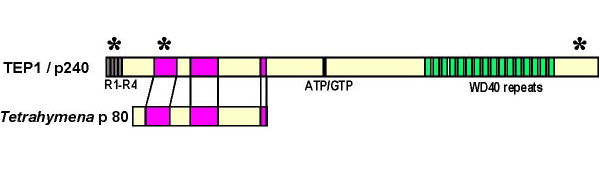
In addition to the major vault protein which comprises approximately 70% of the particle mass, vaults from higher eukaryotes have two high molecular weight proteins; 280,000 and 193,000 daltons. The 280 kDa vault protein (originally called p240 since it migrated on a gel at 240 kDa) has been demonstrated to be identical to a previously described protein thought to be a component of telomerase, TEP1 (Kickhoefer et al., 1999a).
Telomerase is a large nuclear ribonucleoprotein complex that uses an RNA template to catalyze the addition of nucleotides to the ends of chromosomes. TEP1 was first identified based on its homology to the RNA binding domain of the Tetrahymena telomerase subunit p80 (Harrington et al., 1997, Nakayama et al., 1997). Although its role in either complex has not yet been defined, TEP1 has been shown to interact with the mouse telomerase RNA and with several of the human vault RNAs in a yeast three-hybrid assay (Harrington et al., 1997, Kickhoefer et al., 1999a). While purified vaults contain TEP1, they do not possess telomerase activity. Homologous recombination has been used to disrupt the gene encoding mTep1 (Liu et al., 2000). Despite the fact that TEP1 is associated with the telomerase RNA and the telomerase catalytic subunit TERT in vivo, mTep1 deficient mice showed no significant alteration in telomerase activity or telomere length in six generations of mTep1 deficient mice.
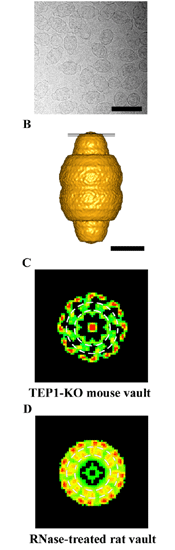
It was recently shown that the levels of the telomerase RNA and its association with the telomerase RNP are also unaffected in mTep1-/- mice (Kickhoefer et al., 2001). The question of whether loss of TEP1 affected the integrity of the vault particle and its associated RNA, vRNA, was also examined. Gross vault morphology appeared to be unaltered in mTep1 deficient mice as observed by both negative-stain (not shown) and cryo-electron microscopy (figure at right, panel A). A three-dimensional reconstruction of mTep1 deficient vaults (panel B) revealed less density in the cap at the location shown by the slice through the vault in panel B. Compare panel C (the mTEP1 deficient vault) to panel D (the RNase-treated wild type vault) and supports the localization of at least a portion of TEP1 to the ends of the vault caps, placing it next to the assigned location of vRNA. Furthermore, the absence of TEP1 completely disrupted the stable association of the vault RNA with the purified vault particle, and also resulted in a decrease in the levels and stability of the vault RNA. Therefore TEP1 has a novel role in vivo as an integral vault protein important for the stabilization and recruitment of the vault RNA to the vault particle.
The presence of 16 WD40 repeats in the carboxyl terminus of TEP1 seemed like a convenient number for this protein to serve a structural or organizational role in the eight-fold symmetric vault particle. although this does not appear to be the case in light of recent data (See Cryo-EM Section). Despite the redundancy of TEP1 in the telomerase complex but not the vault particle, the Kickhoefer et al. (2001) studies reveal the first phenotype associated with the disruption of TEP1 and provide an important first step in elucidating the physiological role of TEP1 in RNA assembly, localization, and RNP function.