Scientists: CryoEM Structure - Part 2
Probing vault structure using recombinant vaults
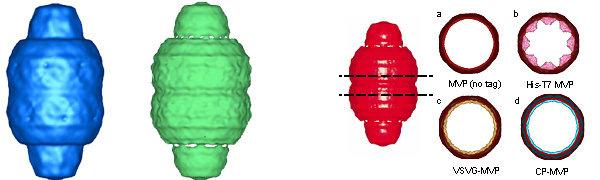
The ability to produce recombinant vaults from altered MVP proteins (see Designer Vaults section) has allowed an examination of vault structure using cryoEM reconstructions and difference mapping. Upon close examination, MVP-only vault-like particles were observed to be somewhat irregular, often containing distorted caps (see the two vaults above left). Co-expression of either VPARP or TEP1 with MVP resulted in vaults composed of two proteins, MVP/VPARP or MVP/TEP1. In addition, co-expression of MVP with both VPARP and TEP1 results in vaults containing all three proteins (Mikyas et al., 2004). Interestingly, TEM examination of particles formed from MVP plus TEP1 and/or VPARP appeared more regular and of overall greater integrity than those formed solely from MVP.
CryoEM particle images of the MVP and MVP-TEP1-VPARP vault-like particle were collected and reconstructed (see figure above left). The MVP-only particles (234 images) only resolve to 37 angstroms (blue vault), whereas 234 images of the vaults containing MVP-TEP1-VPARP proteins resolve to 33 angstroms (green vault). These results indicate that the particles assembled from all three vault proteins are more regular and rigid than the MVP-only particles. These particles are structurally indistinguishable from tissue derived vaults.
Localization of the N-terminus of MVP
The first vaults produced in baculovirus were prepared from an MVP construct that had been tagged at the amino terminus with a 31 amino acid tag (called His-T7). When density slices of the HisT7-MVP-only vaults were compared with wild type mouse vaults, extra density was revealed on the interior of the barrel wall at the central midsection that had not been observed in any previous vault reconstructions (see figure above right, panel b). It was hypothesized that this density might be due to the His-T7 tag added to the N-terminus of each MVP. To test the idea that the extra density at the midsection of the vault might be from the N-terminal MVP tag, reconstructions of recombinant vaults with various length N-terminal MVP tags and vaults with untagged MVP were compared. Three different N-terminal tags were examined including the 31-residue His-T7 tag, a 14-residue VSVG tag, and one 12-residue tag derived from a cysteine-rich peptide (CP-tag). One recombinant vault was also produced without an N-terminal tag. All of the cryoEM reconstructions of vaults with N-terminal MVP tags had rings of density on the inner surface of the vault waist. The density ring for the longer His-T7 tag (31 residues) was considerably thicker than the shorter VSVG and CP tags (14 and 12 residues respectively). The variation in size of the internal density rings is shown in the figure above right (panels a-d). However, in all of the recombinant vault reconstructions the volume of the difference density at the midsection correlated well with the length of the N-terminal MVP tag. Thus it was concluded that this density was indeed from the added peptide tag. This led to the hypothesis that 96 copies of the N-terminal MVP peptide tag meet at the midsection of the vault, with 48 copies attached to MVP subunits forming the top half of the vault and 48 copies attached to MVP subunits forming the bottom half of the vault (Mikyas et al., 2004).
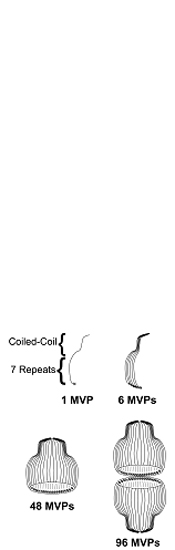
During an examination of the N-terminal tagged vaults, it was noted that one construct was more regular in structure and presumably more structurally intact. This vault, produced from CP-tagged MVP, gave the highest resolution cryo reconstructions as well. In addition, some end on view cryoEM particle images of the CP-MVP vaults had apparent 48-fold rotational symmetry (not shown). A proposed model for the organization of MVP subunits within the vault is summarized in the figure to the right. The model predicts that each MVP monomer is arranges along the outside of a half vault with its amino terminus facing the inside of the particle at the waist and its carboxy terminus at the very top of the vault cap. Furthermore, as each half vault can open into eight petals, the model predicts an intermediate structure with six MVP monomers forming one petal. Eight petals then come together to form a half vault and two halves associate to yield the final structure. The ultimate proof of the model will await an x-ray crystallographic solution (stay tuned!).