Scientists: Delivering Vaults to the Cytoplasm
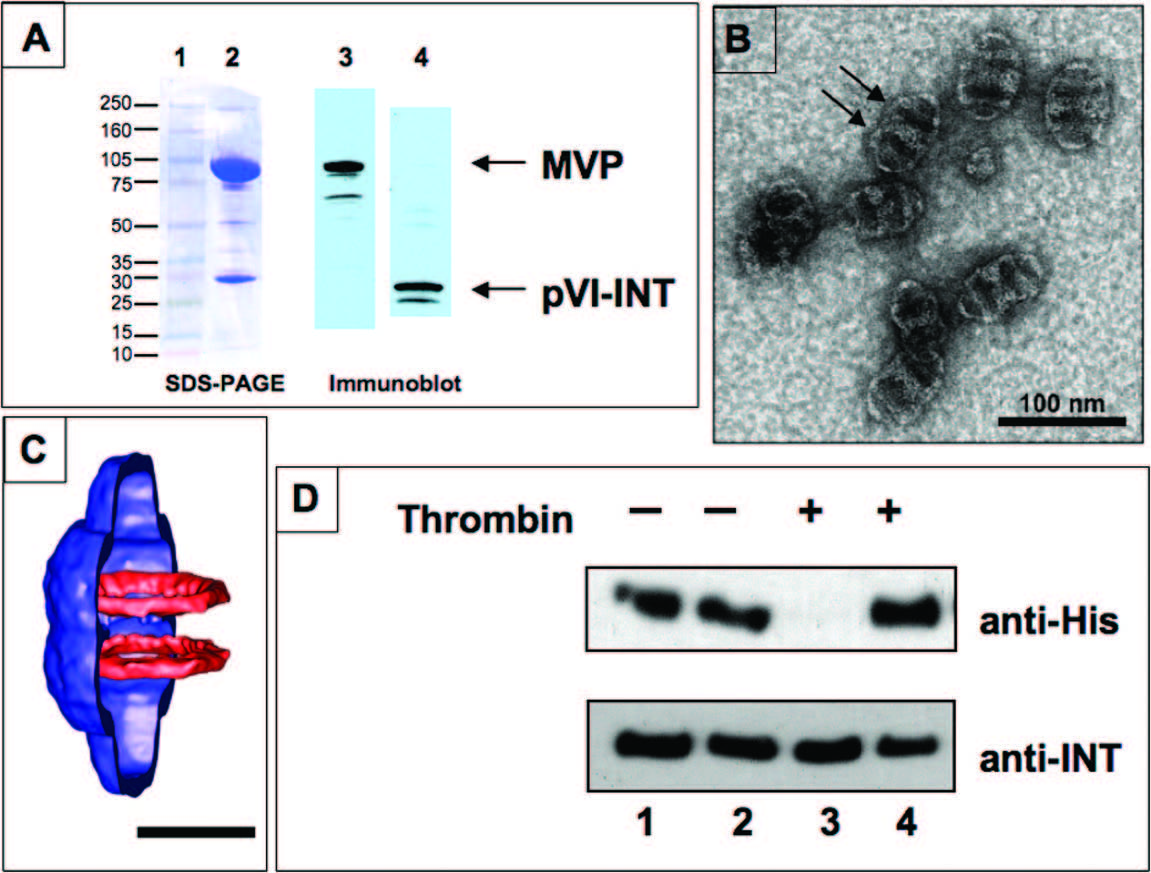
Vaults delivered to cells via cell-surface binding and endocytosis will enter endosomes and presumably be transported to the lysosomal compartment. In order to design the vault as a flexible therapeutic delivery vehicle, it will be important to use the particle to deliver contents to the cyplasmic compartment. In collaboration with Glen Nemerow's laboratory at Scripps Research Institute, the Rome Laboratory has analyzed the ability of vaults to directly escape the endosome using the membrane lytic domain of adenovirus protein VI (pVI) (Lai et al., 2009). Untargeted recombinant vault particles lack demonstrable membrane penetration activity and are thought to enter cells via macropinocytosis or phagocytosis. To explore the feasibility of enabling vault entry into the cytoplasm of target cells, the membrane lytic domain of adenovirus pVI was incorporated into the interior of recombinant vault particles via fusion to the INT domain to make pVI-INT. As see in the figure below in A, recombinant vaults which were packaged with a pVI protein composed of amino acids 34-114 of pVI fused to the VPARP INT domain were purified, and fractionated on SDS-PAGE and stained. The purified vaults are shown stained with Coomassie in lane 2. Immunoblots of purified vaults were probed with either anti-MVP monoclonal antibody (lane 3) or with rabbit polyclonal antisera directed against adenovirus pVI (lane 4). These results confirm the in-frame fusion of Ad pVI with the INT domain and copurification with recombinant vaults. A negative stain TEM image of vaults containing pVI-INT is shown in panel (B) Note the presence of additional protein density (lighter staining) near the waist of the vault barrel, which based on earlier structural studies is the expected location of pVI-INT. This extra density causes an adjacent depression in the particle where stain collects in two bands (indicated by arrows). Lane (C) shows a cropped MVP vault cryoEM reconstruction showing the superimposed density of luciferase-INT in two rings near the waist. Scale bar is 250 nm. (Courtesy of Dr. Phoebe Stewart at Vanderbilt University). This is the expected location of the pVI-INT fusion protein. Panel (D) is an immunoblot of thrombin cleaved proteins. The N-terminus of pVI-INT encodes a 6-His tag and thrombin cleavage site derived from the pET28 expression vector. Purified pVI-INT protein (lanes 1 and 3) or purified recombinant vaults containing pVI-INT (lanes 2 and 4) were either treated with thrombin (lanes 3 and 4) or not treated (lanes 1 and 2). Following digestion, the samples were separated on SDS-PAGE and probed by immunoblotting with either anti-His tag antibody (upper panel) or anti-INT antibody (lower panel). The pVI-INT protein was protected from thrombin digestion in the recombinant vaults but not when treated alone (compare lanes 3 and 4).
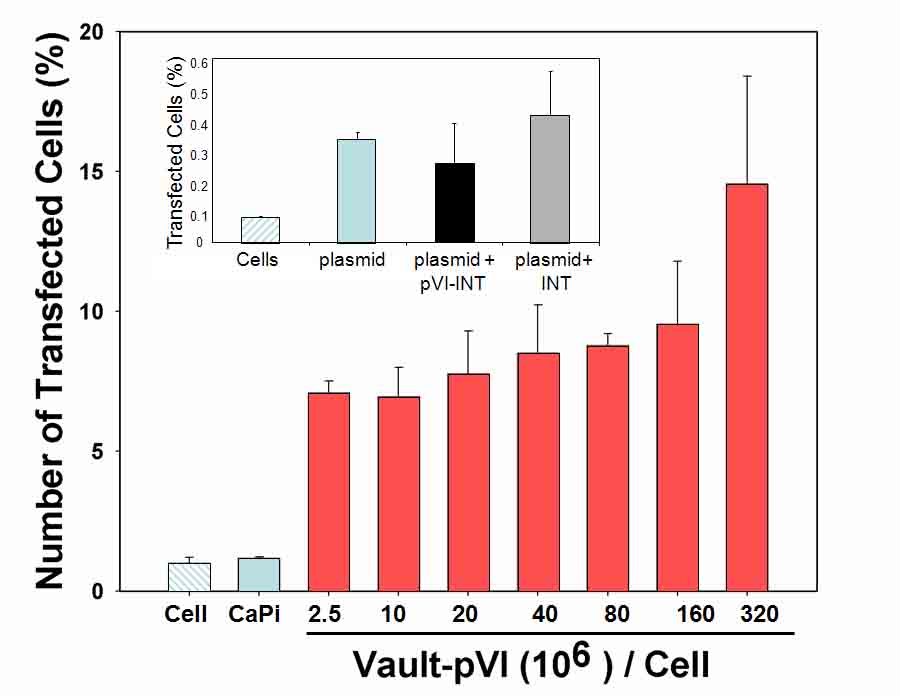
The membrane lytic activity of the pVI domain was retained upon incorporation into vault particles. Moreover, internalization of vault-pVI complexes into murine macrophages (RAW 264.7) promoted co-delivery of a soluble, but non-membrane permeable, ribotoxin or a cDNA plasmid encoding GFP (see Figure below). These findings indicate that vault particles can be modified to enhance the transfer of selected biomolecules across the cell membrane and they offer new avenues for adapting vaults for cell delivery of therapeutic molecules.