Scientists: Dictyostelium MVP Mutants
Disruption of the Dictyostelium mvp genes either individually or in combination, results in altered vault morphology and an impaired growth phenotype under nutritional stress (Vasu et al., 1993, Vasu and Rome, 1995). However, loss of one or both Mvps is not a lethal event. Mvp- mutant lines still produce vaults.
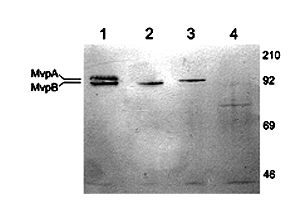
The Western blot above shows wild type Dictyostelium vaults with both MvpA and MvpB proteins (lane 1); mvpA- mutant vaults with only the MvpB protein (lane 2); mvpB- mutant vaults with only the MvpA protein (lane 3) and the double mvpA-B- mutant lacking both MvpA and MvpB proteins (lane 4).
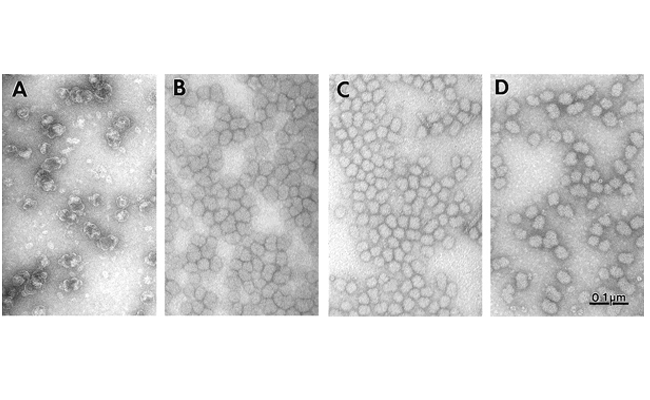
No characteristic lobular vaults were found in either mvpA- (panel B, right), mvpB- (panel C), or the mvpA-mvpB- double mutant line (M7A-B-, panel D) by electron microscopy appearing as fuzzy ovoid structures. This indicates that both proteins are required for normal vault structure (panel A).
The persistence of the ovoid structures in M7A-B-, indicates that MvpA and MvpB are not the sole components of Dictyostelium vaults. Ovoid vaults purified from M7A-B- copurify with a 92 kDa protein which is very poorly detected by available anti-Dictyostelium vault antisera. This protein, designated MvpC, lacks epitopes conserved between not only MvpA and MvpB of Dictyostelium but also Mvps recognized by the anti-rat vault polyclonal antiserum. The failure of MvpA and MvpB cDNA probes to detect MvpC message on northern blots of total RNA from mvpA- and mvpB- cells reflects this divergence. The structural divergence of MvpC suggests functional divergence as well. This protein could represent an additional or partially redundant functional element in the Dictyostelium vault. Both Dictyostelium mvpA- and mvpB- lines show no morphological, developmental or growth defects under permissive culture conditions. However, when passaged into minimal culture media, the mutant lines reach a maximal culture density only one third that of the parental line, JH010.
The data indicate that vault function in the mutant lines must be at least partially impeded. Since losing two Mvps does not seem to increase the magnitude of the growth defect over the loss of a single Mvp, the impediment to vault function is presumably equivalent in both single and double mvp- mutant lines. Whether this impediment is due to loss of Mvp-mediated function or to a reduction in particle structural integrity is unclear. Since MvpC could represent the last remaining component of the M7A-B- vault, this protein will be the next logical target for characterization and disruption. Elimination of MvpC in wild-type or single mvp- lines might simply generate additional mutants with growth defects. However, if MvpC represents an essential functional component of vaults, mvpC- lines might not be viable (Vasu and Rome, 1995).