Scientists: Vaults as Smart Adjuvants
Vault immunogenicity. As a naturally-occurring nanocapsule, the vault particle appears to be an ideal structure to engineer for targeting to tissues. As vaults are highly stable structures in vitro, it is reasonable to propose that the particles will be stable in the bloodstream. Several studies indicate that vaults are non-immunogenic. First, when the Rome Laboratory tried to make anti-vault antibodies in rabbits, they found no antibodies were made to purified vaults. An antigenic response could only be induced when the vaults were hemocyanin cross-linked prior to injection into rabbits (Kedersha, and Rome, J. Cell Biol. 103: 699-709 (1986)). Furthermore, a large panel of human autoimmune antibodies have been screened and the screen failed to find any evidence for antibodies against any of the vault proteins (Rome, unpublished). Immunogenicity studies in mice were recently carried out (Champion., et al., PLoS One. 4: e5409 (2009)). In a nasal spray delivery model, recombinant CP-MVP vaults failed to elicit an antibody response.
A Vault Vaccine Induces Protective Mucosal Immunity. Mucosal immune responses provide superior protection against disease but there are currently no FDA-approved adjuvants capable of stimulating cell-mediated immune responses within mucosal tissues. Perhaps one reason is because mucosal immune responses are generated by stimulating mucosal surfaces. However, mucosal surfaces are hostile environments and immunogenic proteins require added protection for delivery to dendritic cells and induction of immunity. In a recent paper (Champion., et al., PLoS One. 4: e5409 (2009)), Recombinant vaults were tested to see if they could provide such protection by encapsulating an antigen and preserving its functional characteristics even within cells. The internal cavity of the recombinant vault is large enough to accommodate multiple immunogenic proteins. Because vaults are the size of small microbes, a vault particle containing an immunogenic protein should be readily phagocytosed by dendritic cells. Further, recombinant vaults containing proteins are straightforward to produce making vaults a viable vaccine delivery scaffold if they prove facile for generating mucosal immunity. Mucosal immune responses are optimally produced by stimulating mucosal associated lymphoid tissue. For instance, delivery of immunogenic proteins to nasal surfaces stimulates the induction of immune responses within nasal associated lymphoid tissue (NALT). Mucosal immune surfaces are interconnected and stimulation of the inductive site in one mucosal surface, produces an immune response at distant mucosal surfaces. In particular, stimulation of the nasal mucosa induces the appearance of immune cells and antibodies in vaginal surfaces. To test the utility of vaults as mucosal vaccine delivery platforms, an infection that relies on cell-mediated mucosal immune responses for elimination and is a significant burden on health care was chosen; Chlamydia trachomatis infection. Vaginal delivery of the mouse adapted strain of C. trachomatis, C. muridarum, induces a local sexually transmitted infection (STI) similar to human chlamydial STI. C. trachomatis is a prominent cause of STI, with approximately 92 million cases occurring annually and is an instigator of female reproductive dysfunction. T helper immune cells (Th1) must be present within vaginal tissues in order to eradicate infection. However, a vaccine has not yet been produced which induces sizeable Th1 immune responses in mucosal tissues. Therefore, an ideal vaccine would elicit a local antichlamydial Th1 cell response in the reproductive mucosa.
Vaults were modified by the addition of a 33 aa peptide (z) derived from staphylococcal protein A to the C-terminus (hereafter referred to as cp-MVP-z) that binds the Fc portion of immunoglobulins at a site distinct from binding to the Fc receptor (FcR) (Kickhoefer et al. ACS Nano 3, 27-36 (2009)). This vault was packaged with a fusion protein produced from the 366 amino acid coding region of the major outer membrane protein (MOMP) of Chlamydia muridarum (without the signal sequence), fused to the minimal interaction domain (mINT) derived from VPARP (amino acids 1563-1724) by PCR ligation. A schematic is shown below:
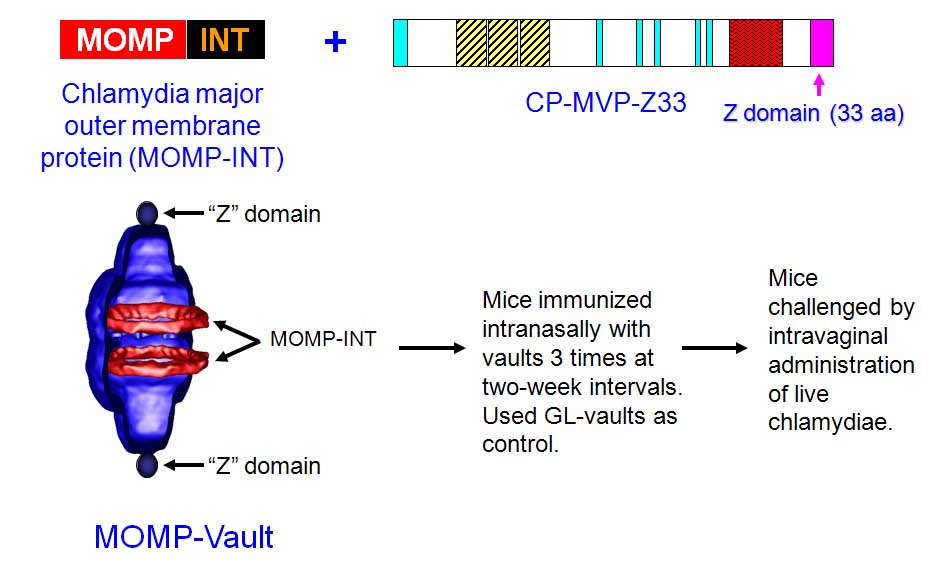
Vaults expressing (cp-MVP-z) or lacking (cp-MVP) the Fc binding peptide were tested for their ability to adhere to mouse Ig isotypes which are generally found in nasal secretions (IgG and IgA) using an ELISA assay. Vaults containing the "z" peptide markedly bound IgG over vaults lacking the "z" peptide. In contrast, regardless of whether or not vaults expressed the "z" peptide, they did not adhere to IgA. Thus, the "z" peptide increases binding of mouse IgG to cp-MVP-z vaults.
To determine if vault "smart adjuvants" containing MOMP could induce mucosal immunity; the C. muridarum genital infection model was used. Mice were immunized by delivering the constructed vaults to the nasal mucosa at 2-wk intervals for total of three immunizations. This immunization regimen was previously shown to reduce infection following intravaginal challenge when soluble Chlamydia plus various adjuvants were delivered together. Mice immunized with 200 mg of MOMP-vaults were estimated to contain approximately 0.2 mg MOMP. As a negative control, mice were immunized i.n. with 200 mg GL-vaults and as a positive immunization control (Live-CM), mice were immunized i.n. with 16 x 10^3 IFU of chlamydiae. Two weeks following the last vault immunization or 4 weeks after the live lung infection, all mice were hormonally synchronized by subcutaneous injection with medroxyprogesterone acetate to normalize infectivity. All mice were challenged 1-wk later by intravaginal administration with 1.56 x 10^5 IFU chlamydiae (the results are illustrated in the Figure below).
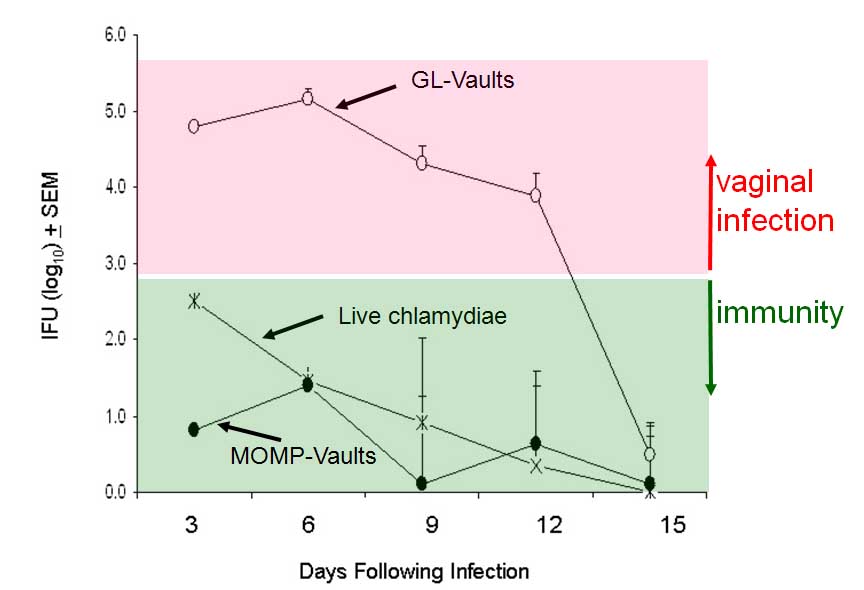
The effect of immunization was first evaluated by monitoring the bacterial burden in vaginal swabs collected every 3 days as reported. Naive, non-immune C57BL/6 mice develop an immune response against C. muridarum and typically clear a genital infection 2-3-weeks later as was observed in mice given GLvaults. In contrast, delivery of 200 mg MOMP-vaults significantly reduced IFU levels isolated from vaginal swabs compared to the group immunized with GL-vaults (see figure above). More importantly, delivery of MOMP within vaults initially reduced bacterial burden approximately 1 log lower than levels seen in mice naturally immune from a lung infection with live chlamydiae (Live CM). This was remarkable protection since in previous studies only MOMP in the native conformation and not recombinant MOMP could achieve this level of protection.
These data demonstrate that vaults containing MOMP induced a powerful mucosal immunity in the absence of co-delivery with a cytokine or using a live microbial vector. Hence, i.n. delivery of MOMP-vaults was effective at inducing protective immunity as demonstrated by reducing the magnitude of infection upon intravaginal challenge with C. muridarum. MOMP-vault immunization enhanced Th1 cell immunity in mucosal tissues. Inflammasome activation facilitates a strong adaptive immune response and promotes the recruitment of immune cells to mucosal tissues Th1 cell immunity in immunized mice was evaluated after vaginal challenge by quantitating the number of CD3+CD4+ cells that secrete IFNc or IL-4 in iliac lymph nodes (ILNs) during peak infection (day 7) and during resolution of infection (day 15). MOMP-vault immunized mice produced Th1 cells and demonstrated the migration of Th1 cells by significantly altering the number of Th1 cells found in ILNs at different times after challenge. In contrast, mice immunized by a previous lung infection with C. muridarum had similar numbers of Th1 cells in ILNs on days 7 and 15. All methods of inducing immunity produced a 10-fold greater number of Th1 cells compared to Th2 cells in the ILNs on days 7 and 15. In addition, a significant increase in a cytokine associated with the presence of T cells (IL-1a) was found in oviduct (OD) homogenates of MOMP-vault immunized mice as early as 7 days after challenge in comparison with immune mice given a lung infection. This finding correlated with increased levels of IFNc in these mice. Likewise, an increase in the level of a chemokine associated with the recruitment of Th1 cells, CXCL10 was also noted. Taken together, these data indicate that protein-containing vaults activate inflammasomes independently of TLR. Inflammasome-activating agents in vivo are capable of producing adaptive mucosal immunity which is characterized in our system by specific antibody production, generation and migration of Th1 cells and protective immunity from a mucosal challenge infection. It appears that protein-containing vaults function as "smart adjuvants" by activating selective properties of DC that stimulate adaptive mucosal immunity. Investigation of the mechanism by which protein containing vaults stimulate mucosal immunity would further development of vaccines.