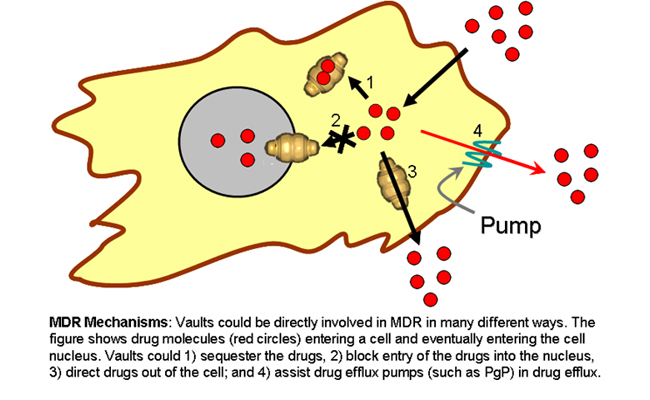
Scientists: Multidrug Resistance
Multidrug resistance (MDR) has been associated with overexpression of transmembrane transporter molecules which act as pumps to remove toxic drugs from tumor cells. These include P-glycoprotein (PgP), multidrug resistance protein (MRP), and breast cancer resistance protein (BCRP). Evidence for an additional mechanism for MDR came from in vitro and clinical studies on an MDR-associated protein originally termed Lung Resistance-related Protein (LRP). Rik Scheper and his colleagues at Free University in the Netherlands, isolated and sequenced a cDNA coding for the LRP gene and found it to be the human major vault protein (Scheffer et al., 1995). Since then numerous studies have been carried out to study the relationship between vaults and MDR. In studies of a variety of human cell lines and tumors MVP levels had the greatest individual value as a marker of in vitro and clinical resistance to chemotherapy (for reviews see Izquierdo et al., 1995 and references therein). While some studies indicated MVP as a prognostic marker of response to chemotherapy, others failed to show any such correlation (Scheffer et al., 2000). Vaults have also been implicated in nucleocytoplasmic transport of cytotoxic drugs. Recently, human vRNAs (hvg-1 and hvg-2) were shown to bind to the drug mitoxantrone (Gopinath et al., 2005). To date vRNA is the only vault component that has been shown to bind directly to a chemotherapeutic compound. Despite the strong connection between vaults and MDR, there have been no studies demonstrating the direct involvement of vaults in imparting MDR to cells except a single study by the Akiyama group (Kitazono et al., 1999). Using the SW620 colon carcinoma cells, they showed that overexpression of MVP upon treatment of cells with sodium butyrate, conferred resistance to doxorubicin due to a rapid efflux of the drug from the nuclei of these cells. They then used MVP-specific ribozymes to knockdown MVP expression in cells, and demonstrated that doxorubicin was more rapidly removed from nuclei of cells that overexpressed MVP compared to those treated with ribozymes. They further showed that knocking down MVP levels resulted in the reversal of the drug-resistant phenotype of the sodium butyrate treated cells. The Rome laboratory has been unable to reproduce these results.
In contrast to the Akiyama group's findings, disruption of the murine MVP gene did not confer sensitivity to cytostatic drugs (Mossink et al., 2002). Neither MVP deficient cells nor MVP knockout mice showed any increased sensitivity to chemotherapeutic drugs. In addition, no differences in daunorubicin influx or efflux kinetics were observed between MVP deficient- and wild type mouse embryonic fibroblasts. Recently, Huffman and Corey (Huffman and Corey, 2005) demonstrated that knocking down the expression of MVP using siRNAs did not change the ability of drug-resistant cells to efflux doxorubicin from the nucleus, and it did not increase the drug-sensitivity of these cells. They also showed that upregulation of MVP in sensitive cells (by sodium butyrate) did not lead to a drug-resistant phenotype. It should be noted that earlier Rik Scheper's group, and recently Erik Wiemer's group showed that mere overexpression of MVP in cells does not confer drug resistance (Scheffer et al., 1993; van Zon et al., 2004). All of these data therefore suggest that despite their upregulation in MDR cells and tumors, vaults do not play a direct role in establishing a MDR phenotype.