Big Kids: TEP1
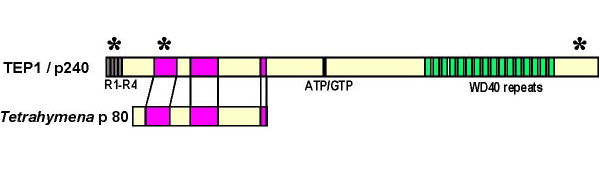
In addition to the major vault protein which comprises approximately 70% of the particle mass, vaults from higher eukaryotes have two high molecular weight proteins; 240,000 and 193,000 daltons. The 240 kDa vault protein was recently demonstrated to be identical to a previously described protein thought to be a component of telomerase, TEP1.
Telomerase is a large nuclear ribonucleoprotein complex that uses an RNA template to catalyze the addition of nucleotides to the ends of chromosomes. Although TEP1 it has been shown to specifically bind the telomerase RNA and the catalytic protein subunit (hTERT), its precise role in the telomerase complex has not been defined. Although TEP1 was shown to be a component of the vault particle and that the protein can specifically bind the vault RNA, vaults did not have any detectable telomerase activity.
The presence of 16 WD40 repeats in the carboxyl terminus of TEP1 is a convenient number for this protein to serve a structural or organizational role in the eight-fold symmetric vault particle. Although the finding of TEP1 in vaults could reflect a simple sharing of protein subunits between telomerase and vaults, the finding may suggest that vaults play some role in telomerase assembly, transport, regulation or function.