Big Kids: Designer Vaults
In 2002 vault research proceeded in a new direction. Vault structure determined by cryoEM clearly demonstrated that the vault particle was a hollow capsule and the experiments of Stephen et al. (2001) indicated that this basic barrel-like structure could be formed entirely from multiple copies of MVP. These findings led to the initiation of a new multidisciplinary collaboration at UCLA between groups in the David Geffen School of Medicine (Rome, Stewart, Eisenberg), the College of Letters and Science (Zink) and the School of Engineering and Applied Science (Dunn, Mobouquette). A proposal was funded by the National Science Foundation (NSF) for formation of a Nanoscale Interdisciplinary Research Team (NIRT).
The overall goal of this NIRT was to develop a flexible, targetable nano-capsule by exploiting the vault. Understanding how the vault capsule could be formed and manipulated would allow molecular manipulations to encapsulate small molecules (drugs, sensors, enzymes, toxins etc.), lengthen and shorten capsule size, and target the engineered nanostructures to specific tissues, cells, or organelles via attachment to receptors, ligands, or fusogens.
Identification of a vault targeting "zip code"
Critical to engineering vaults was the development of a method to package foreign materials into the vault. Accordingly, a strategy was developed to identify an amino acid sequence that, when attached to a foreign protein, could send that protein into the vault. The identification of such a sequence grew from previous studies of the VPARP protein where Kickhoefer et al. (2001) had identified a 162 amino acid sequence found at the C-terminus of the full-length VPARP protein (Figure) as being responsible for the interaction of this protein with MVP. We called this region of VPARP the INT domain (INT because it was responsible for interaction of VPARP with MVP). To determine whether this domain could target a non-vault protein into vault particles, standard molecular biology techniques were used to fuse the INT domain onto the end of a protein originally found in the firefly. This protein, luciferase, is an enzyme responsible for producing the firefly "glow". Attachment of the INT domain to the luciferase enzyme (LUC-INT) and expression in an insect protein expression system (called the baculovirus system) resulted in a protein that retained biological activity. Co-expression of LUC-INT and MVP resulted in its assembly into the inside of the vault. Cryo EM and single-particle image reconstruction revealed that the LUC-INT protein was packaged inside the vault resulting in additional density in two rings in the interior of the particle (Figure).
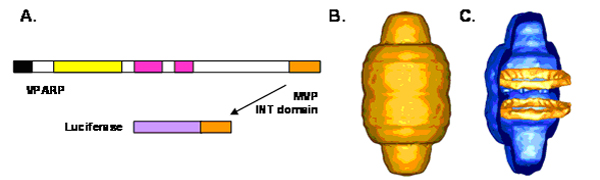
(A) 162 amino acids of VPARP were fused to luciferase. The luciferase-INT fusion protein (LUC-INT) retained enzymatic activity. (B) CryoEM of the MVP-LUC-INT vault reconstruction. (C) Difference map of the LUC-INT/MVP particles minus the MVP-only particles. The difference density is shown in orange superimposed over a half vault from the MVP-only reconstruction. The LUC-INT is seen as two rings of internal density shown in orange. From Kickhoefer et al., 2005.
In addition to targeting luciferase into the particle, we have also demonstrated that the INT domain can target other foreign proteins including the green lantern protein (an analog of the green fluorescent protein) into the particle (Kickhoefer et al., 2005).